Abstract
The mitochondrial architecture is dynamic, undergoing constant fusion and fission. Through mitochondrial fusion, mitochondria form elongated inter-connected networks to promote maximal mitochondrial efficiency. Dynamin Like Protein 1 (Drp1) mediated mitochondrial division ("fission") on the other hand compartmentalizes mitochondria into smaller sized organelles to facilitate cell division and mitochondrial destruction. Recent published pharmacologic studies suggested an intriguing non-mitochondrial role for Drp1 in platelet degranulation. However, the mitochondrial roles of Drp1 in Megakaryocytes (MKs) and platelets have not been addressed. Furthermore, genetic studies are needed to establish a functional role for Drp1 in platelets. Therefore we examined the contribution of Drp1 mediated mitochondrial fission to MK and platelet mitochondrial morphology, platelet numbers, and platelet function.
Using mitotracker labeling of live mitochondria and EVOS fluorescent cell imaging, we examined mitochondrial dynamics and mitochondrial architecture in human and mouse MKs and platelets. In MKs, mitochondria were motile, and formed elaborate interconnected structures. On the other hand, mitochondria in platelets were non-motile, small, and punctate suggesting active mitochondrial fission in late stage MKs and/or platelets.
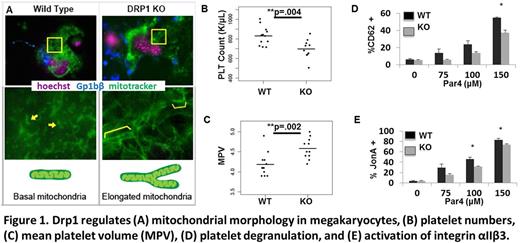
To establish a causative role of Drp1 in regulating MK/platelet mitochondrial morphology, and to assess the role of Drp1 in platelet numbers and function, we generated MK/platelet specific Drp1 null mice (KO). We crossed mice harboring flox (Drp1 fl/fl) sites flanking exons 3-5 of the gene that codes for Drp1 (Dnm1l) with mice harboring Cre recombinase driven by the MK Pf4 promoter (Pf4-Cre). Western blot confirmed that Drp1 protein is abundantly present in platelets from wild type (WT) mice but is absent in platelets from KO mice. Deletion of Drp1 in MKs led to altered mitochondrial morphology: mitochondria in KO MKs formed more hyperextended mitochondrial networks compared to WT MKs (Figure 1A). In Drp1 KO platelets, mitochondria also became less punctate, and more inter-connected compared to WT platelets.
Deletion of Drp1 significantly reduced platelet counts in mice (830±27 vs 698±31 K/μL, p =.004, n=10-12 per group, Figure 1B). Conversely, MPV was significantly increased (4.18±.08 vs 4.58±.08, p=.002, n=10-12 per group, Figure 1C). To assess mechanisms contributing to the difference in platelet numbers, we injected an antibody fab fragment that labels platelets, and tracked platelet life span by flow cytometry. The mean platelet life span of Drp1 KO platelets was modestly, yet consistently reduced (65.1±1.1 vs 56.2±2.3 hours, p=.01, n=4-5 per group). On the other hand, there was not a significant difference in the rate of platelet recovery following antibody depletion of platelets (n=5-6 per group).
Using PAR4 agonist, which activates mouse thrombin receptors on platelets, we found that platelet degranulation is significantly decreased in DRP1 KO platelets compared to WT platelets (Figure 1D, *p<.05, n=4-6 per group). This is consistent with previously published pharmacologic inhibitor studies of DRP1 in human platelets. Unexpectedly, DRP1 KO also reduced activation of the αIIb/β3 fibrinogen binding receptor (Figure 1E, *p<.05, n=4-6 per group), as measured with JonA antibody (binds to the activated heterodimer).
In summary, Drp1 mediated mitochondrial fission regulates mitochondrial morphology in MKs, and disruption of Drp1 in MKs leads to hyperextended mitochondrial networks in MKs and platelets. This corresponds with decreased platelet counts, and increased MPV. Coupled with our observation that αIIb/β3 activation is reduced in DRP1 KO platelets, our findings suggest a novel involvement of DRP1 in regulating platelet numbers and function that may be related to mitochondrial roles of Drp1.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.